

Innovation in Action
The MARIO study
Investigating ibrexafungerp as a non-azole, oral step-down for patients with invasive candidiasis and candidemia
MARIO is an innovative study to investigate oral ibrexafungerp as a potential step-down antifungal therapy following IV echinocandin for these life-threatening infections.
If successful, the MARIO study will give healthcare providers the opportunity to step-down their patients to a non-azole oral therapy that retains the MOA (glucan synthase inhibition) of the IV-only echinocandins, which are the gold standard for treatment of Candida infections, potentially allowing for faster discharge from the hospital.
Clinical trials can provide patients with access to the latest, most advanced investigational therapies.
How the MARIO trial works
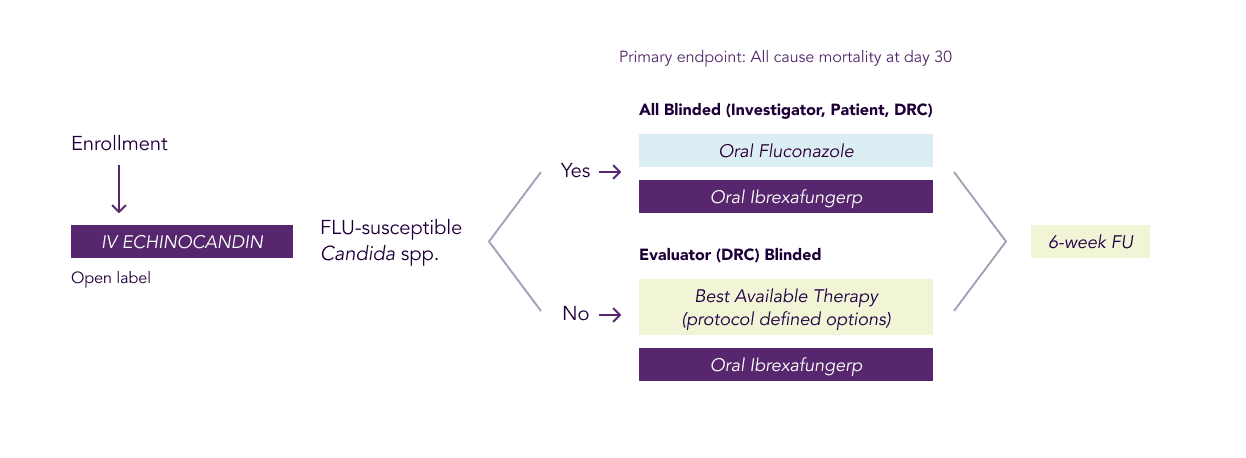
As part of the trial, eligible patients with IC and/or candidemia will receive an initial course of treatment with the current standard of care, an intravenous (IV) echinocandin.
Patients in the study who meet the step-down criteria will then be treated according to the nature of their infections. For fluconazole susceptible isolates, patients will receive either oral ibrexafungerp or oral fluconazole (double-blinded). For fluconazole non-susceptible isolates, patients will receive either oral ibrexafungerp or the best available therapy (evaluator-blinded). A step-down therapy is given usually when a patient is stable and ready to go home. The step-down treatment allows for additional flexibility as the patient continues treatment and may enable them to go home from the hospital.
About the investigational treatment
Ibrexafungerp is an antifungal agent and the first representative of a novel class of structurally-distinct glucan synthase inhibitors, triterpenoids. This agent combines the well-established activity of glucan synthase inhibitors with the flexibility of oral formulation. Ibrexafungerp has demonstrated broad-spectrum antifungal activity, in vitro and in vivo, against multidrug-resistant pathogens, including azole- and echinocandin-resistant strains.
The U.S. Food and Drug Administration (FDA) granted Qualified Infectious Disease Product (QIDP) and Fast Track designations for the oral and IV formulations of ibrexafungerp for the indications of invasive candidiasis (IC), including candidemia, and invasive aspergillosis (IA) and has granted Orphan Drug Designation for the IC and IA indications. The European Medicines Agency (EMA) has granted ibrexafungerp Orphan Medicinal Product designation for the IC indication.
You can learn more about ibrexafungerp here.
If you want to learn more about the MARIO trial click here.

Why is the MARIO study innovative?
- MARIO is the first study to compare efficacy of oral step-down therapies after treatment with an active IV antimicrobial. In addition, the arm of the study that compares oral ibrexafungerp to best available therapy speaks to the unmet medical need for a non azole oral alternative particularly for patients with azole resistant isolates.
- Treatment guidelines for IC recommend a glucan synthase inhibitor (IV echinocandin) as preferred first-line therapy. The study is the first to offer patients an opportunity to continue therapy with an oral glucan synthase inhibitor for the treatment of IC or Candidemia.
- If approved, oral ibrexafungerp would be the first non-azole oral therapy available for invasive candidiasis and would enable an IV-to-oral transition with a consistent mechanism of action.
About invasive candidias
IC is a systemic infection typically affecting patients with an underlying disease or condition, such as those who are post-transplant, post-surgery, in intensive care units, or those receiving immunosuppressive treatments. IC has a high mortality rate and often involves the blood and other organs, such as liver, spleen and bone.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF EMBRYO-FETAL TOXICITY
- BREXAFEMME is contraindicated in pregnancy because it may cause fetal harm based on findings from animal reproductive studies.
- For females of reproductive potential, verify that the patient is not pregnant prior to initiating BREXAFEMME treatment. Reassessing pregnancy status prior to each dose is recommended when BREXAFEMME is used monthly for 6 months for reduction in the incidence of recurrent vulvovaginal candidiasis (RVVC).
- Advise females of reproductive potential to use effective contraception during treatment of vulvovaginal candidiasis (VVC) and throughout the 6-month treatment period for reduction in the incidence of RVVC with BREXAFEMME and for 4 days after the last dose.
CONTRAINDICATIONS
BREXAFEMME is contraindicated in:
- Pregnancy
- Patients with hypersensitivity to ibrexafungerp
WARNINGS AND PRECAUTIONS
RISK OF EMBRYO-FETAL TOXICITY
BREXAFEMME use in pregnancy may cause fetal harm based on animal studies. Prior to each dose of BREXAFEMME in patients of reproductive potential, verify patients are not pregnant and advise them to use effective contraception during treatment for VVC and throughout the 6-month treatment for reduction in the incidence of RVVC and for 4 days after the last dose.
ADVERSE REACTIONS
Most common adverse reactions (≥2%) reported in BREXAFEMME clinical trials:
- VVC: diarrhea, nausea, abdominal pain, dizziness, and vomiting
- RVVC: headache, abdominal pain, diarrhea, nausea, urinary tract infection, and fatigue
DRUG INTERACTIONS
Concomitant use of strong CYP3A inhibitors (e.g., ketoconazole, itraconazole) increases the exposure of ibrexafungerp. Reduce BREXAFEMME dosage with concomitant use of a strong CYP3A inhibitor to 150 mg twice daily for one day.
Concomitant use of strong and moderate CYP3A inducers (e.g., rifampin, carbamazepine, phenytoin, efavirenz, etravirine) may significantly reduce the exposure of ibrexafungerp. Avoid concomitant administration of BREXAFEMME with strong or moderate CYP3A inducers.
USE IN SPECIFIC POPULATIONS
There is a pregnancy safety study for BREXAFEMME. If BREXAFEMME is inadvertently administered during pregnancy or if pregnancy is detected within 4 days after a patient receives BREXAFEMME, pregnant women exposed to BREXAFEMME and healthcare providers should report pregnancies by calling 1-888-982-7299 or going to https://www.brexafemmepregnancyregistry.com.
Please see full Prescribing Information and Medication Guide, including BOXED WARNING.
Innovation with impact
We are fighting life-threatening infections with innovative science.
Learn More