

Our Science
Advancing dynamically
from molecule to medicine
Committed to protecting the world against
dangerous and difficult-to-treat infectious diseases
Next-generation triterpenoid antifungals
(the fungerps)
When working in the field of life-threatening infectious diseases, you are racing against rapidly evolving pathogens.
Since our company’s inception, we have focused on getting in front of changing fungal infections that are growing resistant to existing agents and are becoming increasingly deadly.
Today, our research continues as we explore the next-generation antifungals with potentially transformative fungicidal potency against yeasts and molds.
Our eyes are on the future and what’s next.

How do fungerp compounds work?
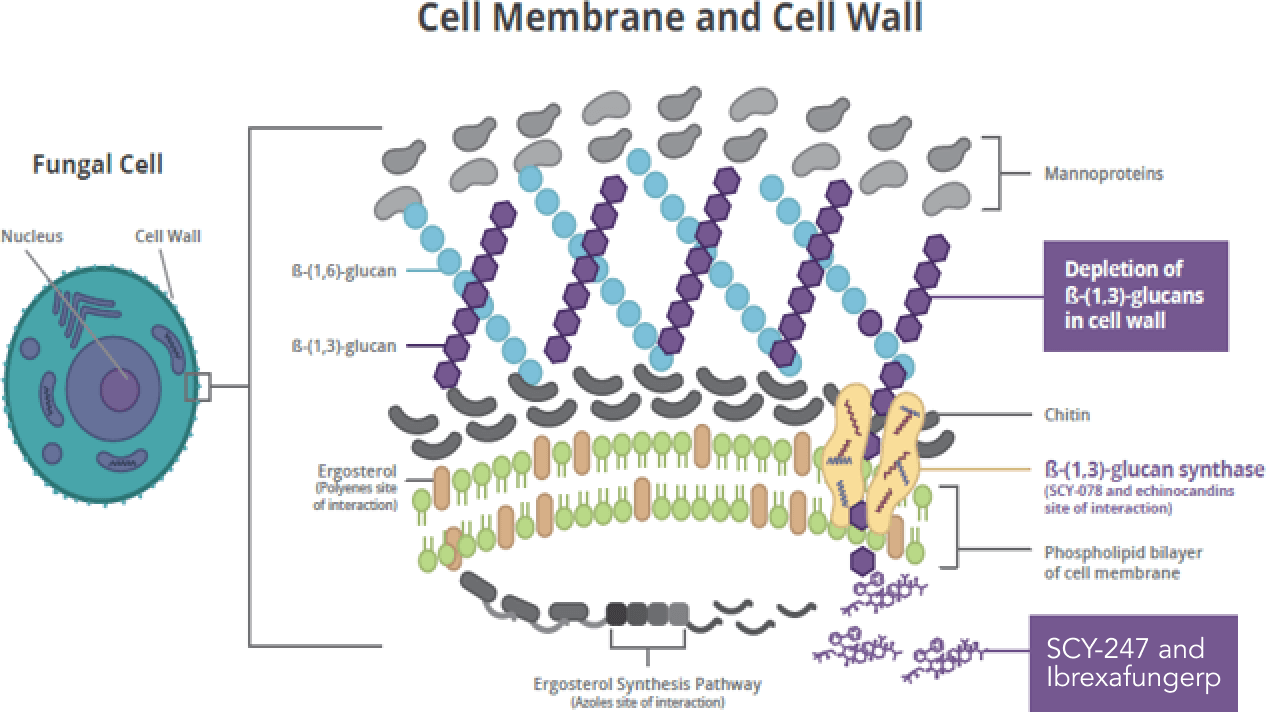
- Validated Mechanism of Action – inhibition of glucan synthase
- The MOA (glucan synthase inhibition) has been previously validated as a clinically relevant target by the echinocandins
- Differentiated binding vs. echinocandins
- Due to the different structure and enzyme-inhibition properties, fungerps retain activity against the majority of echinocandin-resistant strains
- Inhibition of the synthesis of β-(1,3)-D-glucan, an essential component of the fungal cell wall of many pathogenic fungi, results in cytological and ultrastructural changes in the fungi compromising their ability to survive
- Disruption of the fungal cell wall is fungicidal against Candida spp.
- No cross-resistance with azoles
- The fungal cell wall is a unique target not encountered in mammalian cells, which provides for the good safety profile of the fungerps and other glucan synthase inhibitors (limited risk for “off-target” effects, such as human cell toxicity)
How are the fungerps different?
The structures of the fungerp class of antifungals and echinocandins are very different, allowing for:
- Different enzyme-inhibition properties than the echinocandins
- Most gene mutations (FKS) that confer resistance to echinocandins do not affect the activity of fungerps
- Administration orally and IV (echinocandins are only available IV)
- Extensive distribution into key tissues associated with fungal infections, often achieving tissue concentration severalfold above the plasma concentrations (echinocandins have a limited tissue distribution achieving tissue concentration at or below plasma concentrations)
The MOA of the novel fungerp antifungals is different from the azoles and polyenes, allowing for:
- Retention of activity against azole and polyene-resistant strains
- More favorable safety profile
- Lower risk for drug-drug interaction (vs. azoles)
- Retained activity at vaginal pH (4.5)
- Synergistic antifungal activity when combined with azoles or polyenes, particularly relevant for the treatment of invasive aspergillosis
Our focus for the fungerp platform
Interested in our clinical trials?
Find out more about what trials are going on presently.
Learn MoreOur Data
SCYNEXIS regularly presents key infectious disease study data at top medical meetings.
Learn More